Investigator site selection is a crucial element of clinical development, but one dogged with challenges and inefficiencies.
Dr Gen Li, Founder & President of Phesi, is on a quest to bring transparency and objectivity to investigator site selection. Here he explains how advanced clinical data analytics is changing the game, helping sponsors to find the ‘needles in the haystack’ – the perfect investigator sites globally for their specific trials.
Although it is a comparatively short part of the wider clinical development process, ensuring that clinical trials take place at the highest-enrolling investigator sites is vital to recruiting and retaining patients with the required profile for a trial – ultimately accelerating the path to drug approval and improving ROI for sponsors.


16% of investigator sites contribute 50% of patients
Rapid progress in precision medicine has stratified patient populations and increased understanding of disease mechanisms, but the same level of precision is not yet reflected in investigator site selection.
A recent Phesi analysis of patient enrolment data from oncology trials revealed a huge disparity between investigator sites.
Almost one fifth of trial sites contribute just 3% of patients, and 16% of the best performing sites contribute almost half of patients. The analysis demonstrated that a lack of precision in site selection and a reliance on over-burdened sites is significantly impacting the speed and cost of clinical development.
Common mistakes in investigator site selection
But why do trials end up selecting investigator sites that provide little or no patient data?
Selection of site list – typically, an investigator site list is produced from internal nominations, suggestions from development partners (including partner CROs), or even simply based on familiar locations or institution names. This approach doesn’t take into account all the available data that could confirm the viability of these investigator sites. It can also lack transparency if sponsors are not aware of exactly how each suggestion was decided upon.
Lack of nuance – Even third-party selection tools used to augment these nominations often take an over-simplified approach based on tiered lists. Without added nuance, these lists could result in most sponsors in a disease area selecting the same high-ranked investigator sites, which could quickly become overwhelmed and unable to recruit enough patients even if they were the right sites to enrol patients defined by the study design.
Feasibility questionnaires – feasibility questionnaires are sent to the considered investigator sites to assess their suitability for a trial. But this extremely time-consuming and costly activity, rarely delivers meaningful analysis. It may also be unnecessary – as huge amounts of historical and current investigator site data often already exists that can be used to gather the same insights.
Data-driven investigator site selection
A data-driven approach to the trial design based on up-to-date, accurate, real-world data, would allow for a deep dive into the profile of each site and offer a more robust quality assurance step.
Considerations include:
- The number and type of trials the investigator site has been involved with in the past
- The current workload
- Which indications, drugs, treatment modalities and methodologies an investigator has worked with
- Their levels and speed of enrolment in comparison with their peers
- Access to the right patient population in varied demographics as being defined by the protocol
- Speed of investigator site activation
- Quality of data produced
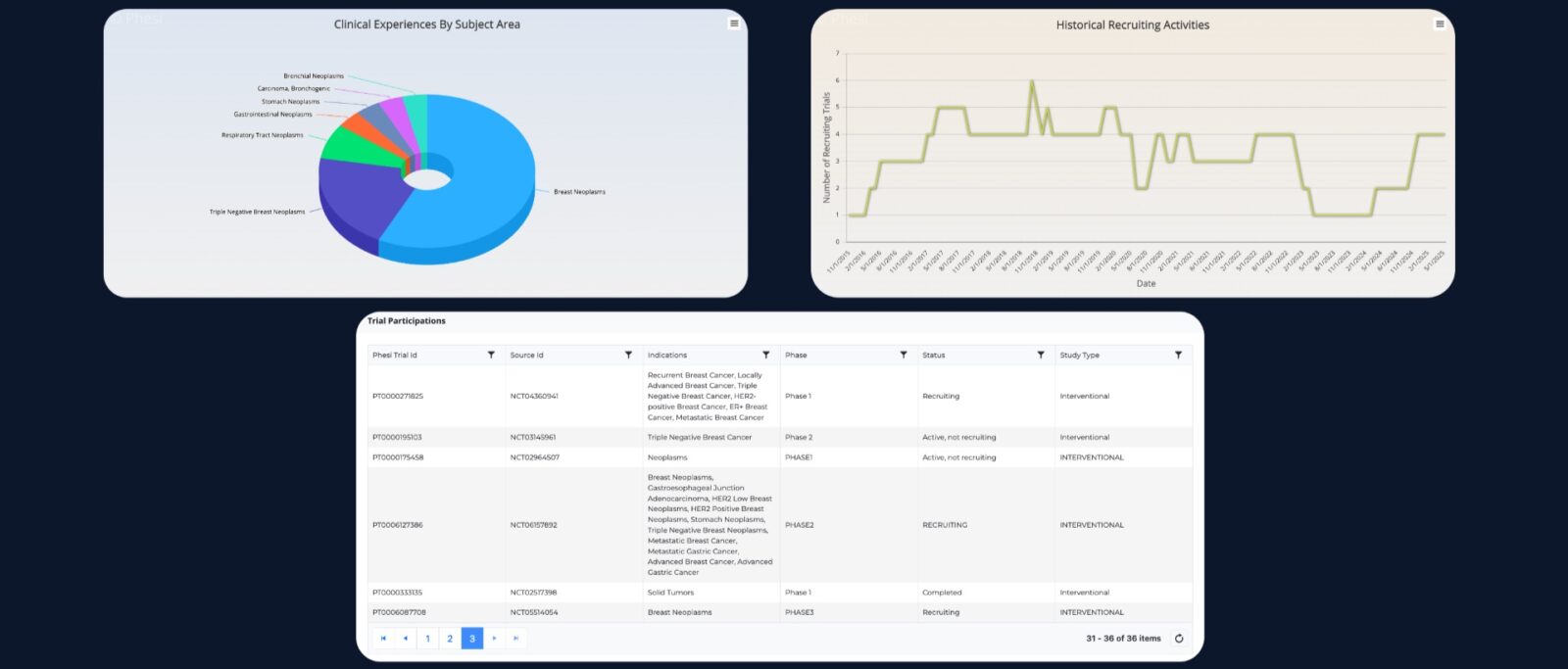
An example profile of an investigator using this data-driven approach is below. This profile draws upon information from the data lake in Phesi’s AI-powered Trial Accelerator Platform, and has been compiled via the Investigator Site Profiler software.
Armed with this kind of information, sponsors can eliminate 20%-30% of investigator sites from lists where there isn’t sufficient evidence to justify inclusion.
This allows for a ‘needle in the haystack’ approach – highlighting the investigator sites that might not be the most obvious choices in the context of the wider disease area, but are the perfect fit for a specific trial.
And since these lists can often include several hundred potential sites, this will also result in significant time and cost savings.
A precision-led future
Clients are under immense pressure to boost ROI on every project, while achieving more with fewer resources and reducing patient burden. Rethinking key feasibility assessment aspects of the clinical development process will improve investigator site selection and have huge benefits for the entire development cycle. Enabling faster enrolment would substantially reduce the cost of activation and monitoring.
With a precision-led approach, clinical development organisations can design smarter trials that lead to faster cures.